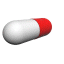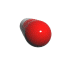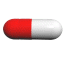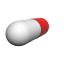

The U.S. Food and Drug Administration (FDA) approved the first generic versions of Cymbalta on December 11, 2013. The prescription medication, duloxetine delayed-release capsules, is used to treat depression other psychological conditions. Signs and symptoms of depression include depressed mood, loss of interest in once-pleasurable activities, significant appetite or weight change, insomnia or hypersomnia, restlessness/pacing, increased fatigue, impaired concentration, feelings of worthlessness or guilt, and thoughts of suicide or suicide attempts.
Aurobindo Pharma Ltd., Dr. Reddy’s Laboratories, Ltd., Lupin Ltd., Sun Pharma Global FZE, Teva Pharmaceuticals USA, and Torrent Pharmaceuticals Ltd. have received approval from the FDA to market duloxetine in various strengths. According to Kathleen Uhl, M.D., acting director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research, “consumers can be assured that these FDA-approved generic drugs have met our rigorous standards.” In other words, the generic versions of Cymbalta, as with any generic prescription drug, will “have the same high quality and strength as brand-name drugs.” With the impending availability of the generic versions of Cymbalta, the medication will be more widely available to patients. The availability of generic duloxetine and any additional information can be obtained directly from the manufacturers.
Source: FDA approves first generic versions of antidepressant drug Cymbalta, December 11, 2013